Too much drug information can be dangerous, especially when it does not help to guide practice decision. Recently, a nurse practitioner asked me if there was any interaction between Tamiflu and warfarin. My quick answer was no. But when I looked it up at Lexicomp (an online drug reference), this was what I found:
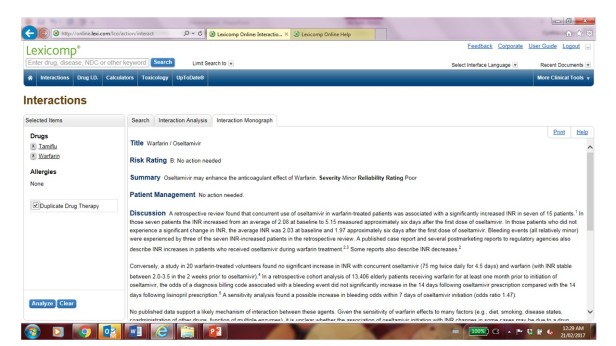
If one is not meticulous to read through the information, one may think there is a relevant drug interaction to be concerned with. Yet if you read carefully, the information provided is to describe some inconsistent findings from the literature. One study cited there was a significant drug interaction; other studies have not come to the same conclusion. Given there is no clear explanation for the interaction between warfarin and Tamiflu and the information has poor reliability, the recommendation is that “no action needed”.
So why bother sharing such useless crap in the first place!
Many pharmacists feel it is necessary to provide prescribers with all types of drug interaction warnings regardless of the severity, the reliability or the relevance of the interactions. They argue that there may be a liability issue if the information is not shared.
On the other hand, I have heard prescribers requesting pharmacies not to fax these notifications because they are either aware of the interactions or these notifications are clogging up their fax machines or drowning them with unnecessary paperwork.
Not all drug interactions are clinically relevant. Some are insignificant, others are easily managed but some interactions truly require the clinicians to intervene. That’s why it is important to understand that each drug interaction often comes with a risk rating score to guide if further action is necessary.
According to Lexicomp:
- Each drug-drug interaction is assigned a risk rating of A,B, C, D, or X.
- In general, risk rating of A and B are of academic but not clinical concern, whereas C, D, or X indicate situations that may require a clinician’s attention.
- A = No known interaction
- B = No action needed (e.g. Tamiflu and warfarin)
- C: Monitor therapy (e.g. ACE-Inhibitor and Septra – may increase risk of hyperkalemia)
- D: Consider therapy modification (e.g. warfarin and Aspirin – may increase risk of bleed)
- X: Avoid combination (e.g. citalopram and quetiapine- both are QT interval prolonging drugs)
However, the interaction alert is provided without regard to the patient’s history or status. For instance if the patient has been on both citalopram and quetiapine for a long time and stable with no concern with QT interval prolongation, continuing the combination may be reasonable whereas abruptly discontinuing one of the medications may be dangerous and inappropriate. Further, if a patient is stable on an ACE-inhibitor but was recently started on Septra for a urinary tract infection, it may be more appropriate to change the antibiotic to something different, especially if the patient’s potassium level cannot be closely monitored.
So when one is confronted with a drug interaction alert, one should be asking the following questions:
- Is the interaction involved a new medication for the patient? Or the patient has been stabilized on this combination of medications for a long time?
- Consider the severity of the interaction? Is it minor or major?
- Verify the reliability of the information? If the information has good reliability, it may warrant further monitoring. But if the information has poor reliability, it may be taken with a grain of salt.
- Try to understand the nature of the interaction? If there is an absorption concern, it may not be relevant if the medication is given intravenously. If the interaction may increase the risk of adverse side effects or reduce the efficacy of the medication, can it be easily managed or monitored (e.g. checking the INR, clinical assessment, etc)?
- If it is more reasonable to monitor the potential drug interaction then to change the medication, ensure to monitor and follow up at the right time frame:
- For example, increased sedation as a result of adding a strong opioid will be noticeable after 24-28 hours, whereas worsening of side effects from altering the metabolism via the P450 system may take a week to notice the effect.
Drug interaction can be a complex topic to understand – some interactions are theoretical but not clinically significant while other interactions can be relevant and need clinicians to intervene. Hopefully, these questions along with a good understanding of the risk rating scale will help clinicians to better manage drug interactions for their patients.
See below for a detailed description of the risk rating score:
|

I learn a lot from your blog, as I read up on every drug interaction for anything I’m taking, and tend to take the least amount of anything prescribed, just in case.
LikeLiked by 1 person
Thank you! I am also a minimalist and like to avoid or minimize drug interactions. But sometimes the benefits may outweigh the risks and we just need to “manage” the interactions.
LikeLike
What is your area of expertise? Are you a pharmacist? A physician? A nurse? Just curious…nan
LikeLiked by 1 person
Yes pharmacist. 🙂
LikeLike